Titanium
Last updated:7 September 2023
Introduction
Humans have used metals such as iron and copper for thousands of years. By contrast, titanium is a relatively 'modern metal', having been first produced commercially as recently as the 1940s into products such as wires, sheets and rods. Since then, titanium has come to be used for many different and important purposes due to its light weight (low density) and high strength.
However, unless you have a heart pacemaker, ride an expensive bicycle, or play a sport that uses titanium-based equipment, your most likely contact is not with titanium metal but with titanium dioxide - in paints, paper, toothpaste or sunscreen, or when you've eaten any food with colouring, like Smarties. If you want to know when you're eating titanium dioxide, look out for food colouring number 171 on the packet.
Military aircraft such as the F-22 and UH-60 Black Hawk helicopter contain large quantities of titanium. As humans continue to explore space, titanium is likely to be needed in increasing amounts for the rocket engines, pressure vessels and structural parts of space vehicles.
Properties
Titanium is a lightweight, strong and rust-resistant silver-white metal. Pure titanium is quite soft but titanium alloys are extremely strong (even stronger than steel and aluminium). Titanium has a very high melting point and is non-toxic. Titanium dioxide is one of the whitest, brightest substances known. Because of its opaque and reflective properties it is used as a pigment in paints, plastics and paper. Titanium dioxide is also used in sunscreen because of its ability to reflect UV light.
The Properties of Titanium
| Chemical Symbol | Ti |
|---|---|
| Ore | Heavy mineral sands - rutile and ilmenite |
| Relative density | 4.5 g/cm3 |
| Hardness | 6 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Melting point | 1668°C |
| Boiling point | 3287°C |
Rutile
Rutile is named after the Latin word rutilus, meaning red. The mineral rutile is a rich source of titanium dioxide and is usually found as part of mineral sand deposits. It is also used for the production of titanium metal.
The Properties of Rutile
| Chemical Symbol | TiO2 |
|---|---|
| Colour | Brownish red |
| Hardness | 6-6.5 |
| Relative density | 4.23 g/cm3 |
Ilmenite
Ilmenite is named after the Ilmenski mountains in Russia where the mineral was first discovered. Ilmenite is slightly magnetic, which means that magnets can be used to separate it from other minerals in sand deposits. It is able to withstand extreme temperatures and is used in the steel industry to line blast furnaces. Ilmenite's hardness also makes it useful as an abrasive. Ilmenite is the main source of titanium dioxide which is used in paints, fabrics, plastics, paper, sunscreen, food and cosmetics.
The Properties of Ilmenite
| Chemical Symbol | FeTiO3 |
|---|---|
| Colour | Black/grey |
| Hardness | 5-6 |
| Relative density | 4.7-4.8 g/cm3 |
Uses
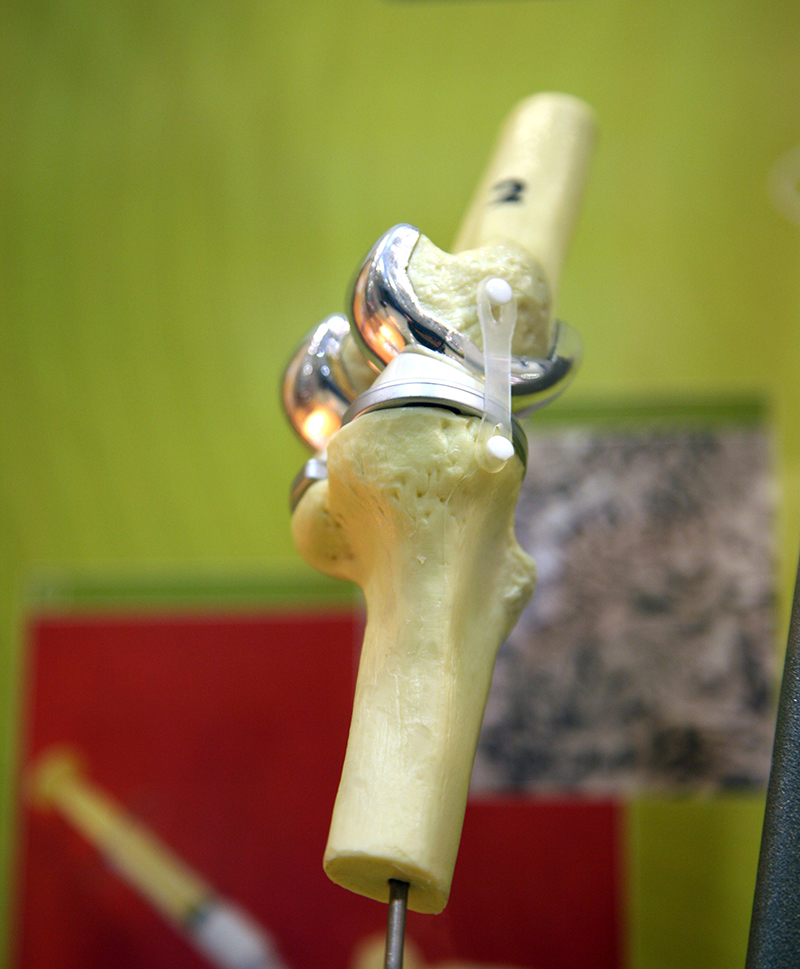
Almost all rutile and ilmenite is processed into non-toxic white titanium dioxide pigment for use in the manufacture of paints, plastics, paper, ink, rubber, textiles, cosmetics, leather and ceramics. Titanium dioxide pigment has excellent brightness and high opacity for good hiding power (e.g. in paint for covering undercoats) and has replaced lead carbonate pigments. Ilmenite is used as a fluxing agent in blast furnace feeds and as a sand-blasting abrasive. Rutile is used in fibreglass, chemicals and as a coating on welding rods. Rutile is also used to produce light, strong, corrosion-resistant titanium metal for use in aircraft, spacecraft, motor vehicles and desalination plants. Titanium metal is biocompatible with the human body and is thus used for surgical implants such as knee and hip replacements.
| Use | Description |
|---|---|
| Titanium metal |
Titanium's light weight combined with great strength, rust resistance and very high melting point make it ideal for use in aircraft engines, spacecraft, missiles, cars, sports equipment (such as racing yacht parts, golf clubs, tennis racquets and bike frames), wrist watches, underwater craft, and general
industrial equipment. Its non-toxicity also makes it useful for surgical implants such as pacemakers, artificial joints and bone pins. Titanium is also used to manufacture chlorine. |
| Titanium dioxide |
As titanium dioxide is such a white pigment and its reflective properties add richness/brightness to colours, and it provides UV protection, it is used in paints (replacing the use of lead), lacquers, paper, plastics, ink, rubber, textiles, cosmetics, sunscreens, leather, food colouring, and ceramics. It is also used as a coating on welding rods. |
| Titanium tetrachloride | This compound fumes in moist air, making a dense white smoke, so is used for smokescreens and skywriting. |
History
Titanium was named in 1795 by a German chemist, after the Titans of Greek mythology who were very strong. Work in the 1940s demonstrated a method for titanium production on a laboratory scale, however, it took nearly a decade more before it could be adapted for large-scale production. In 1948, DuPont opened the first significant manufacturing operation in America. During the Cold War the Soviet Union started to use titanium for military equipment and the USA closely followed with a particular focus on aircraft.
Formation
Rutile and ilmenite originally grew as crystals in igneous rocks such as granite, pegmatite and basalt and some metamorphic rocks. Over millions of years, these rocks were weathered and eroded forming small grains of quartz and other minerals including rutile and ilmenite. These grains were washed down to the sea by heavy rainfall and fast flowing streams and became part of the coastal sands. The relative density of common sand minerals such as quartz is around 2.65 g/cm3, however, heavy minerals found in mineral sands have a relative density of between 4 and 5.5 g/cm3. As waves washed up and down on the beach they carried the lighter quartz grains with them back out to sea, leaving the grains of the heavy minerals on the beach. Wind also helped to concentrate the heavy minerals by blowing away the lighter quartz sand. Repeated many times over millions of years, these processes eventually created large deposits of mineral sands.
As the sea level fell and rose over geological time, the shoreline moved further inland and then back again. As this happened, the deposits of mineral sand were covered by more sediment and built up or were eroded and redeposited elsewhere. This is why we sometimes find mineral sand deposits many kilometres inland and as much as 50 metres below the surface.
Resources
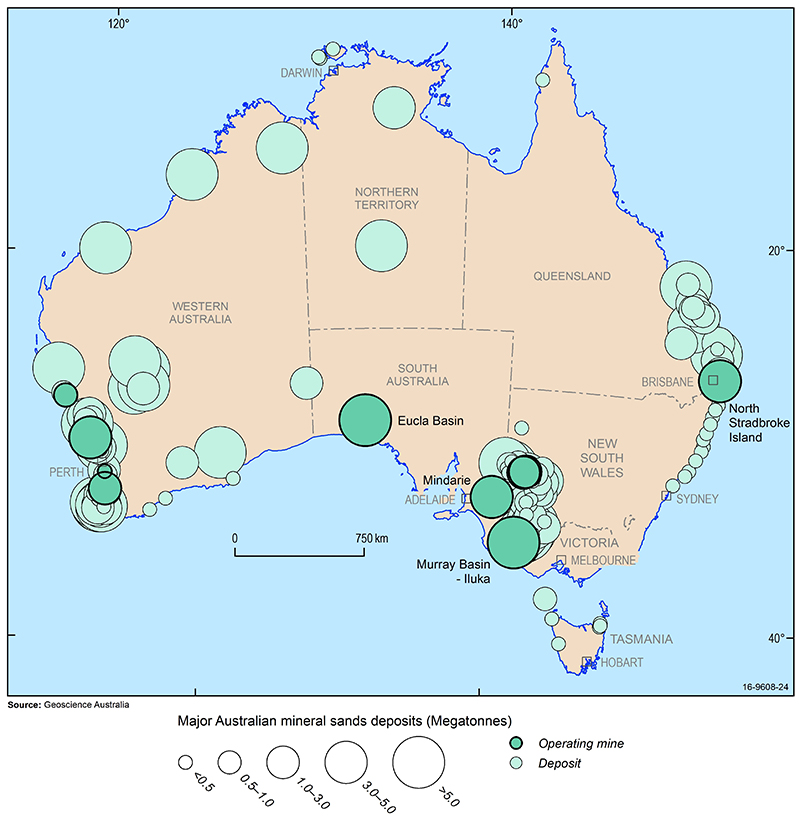
Rutile and ilmenite are found in mineral sand deposits associated with modern and ancient beaches and dunes on the east, west and southern coastlines of Australia. Mineral sands deposits occur along the coast of eastern Australia from central New South Wales to Cape York in Queensland. Large relic or old beach deposits are found as far inland as Ouyen in Victoria (Wemen, Bondi, Kulwin deposits) and southwestern New South Wales (Ginkgo, Snapper deposits). Deposits also exist in the Eucla Basin around the Great Australian Bight in South Australia and Western Australia. In Western Australia, deposits are distributed from the southern tip of the state to Geraldton and are located at the present coastline or as relic deposits up to 35 km inland.
Australia is rich in mineral sand resources but, because they are mainly located at or near the coast, their mining competes with other land uses such as agriculture, national parks, urban or tourist development and recreation. Allocation of land to other uses has rendered some mineral sands resources inaccessible to exploration or mining. Geoscience Australia estimates that around 44% of ilmenite and 26% of rutile are unavailable for mining. Deposits in this category include Moreton Island, Bribie Island and Fraser Island, the Cooloola sand mass, the Byfield sand mass and the Shoalwater Bay area in Queensland as well as the Yuraygir, Bundjalung, Hat Head and Myall Lakes National Parks in New South Wales.
Throughout the late 1990s, a large number of coarse-grained strandlines were identified in the Murray Basin, which occurs within New South Wales, Victoria and South Australia. Over 100 million tonnes of heavy mineral sand concentrates were outlined. Large resources of fine-grained mineral sands deposits (referred to as WIM-type deposits) occur in the Horsham region of Victoria.
In Australia, Victoria hosts the greatest share of known rutile resources with just over 50% while Western Australia hosts just under 50% of known ilmenite resources. Internationally, Australia hosts around 40% of global rutile resources (compared to Kenya (24%), South Africa (15%) and India (14%)) and 19% of global ilmenite resources (compared to China (27%), India (11%) and South Africa (9%)). Australia accounts for around 13% of the global production of ilmenite. Other leading ilmenite producers include China (16%) and Vietnam (10%). Australia produces around 30% of the world's rutile followed by Sierra Leone (23%).
Mining
Although mineral sand deposits were noticed back in 1870, sands were first mined in Australia in the 1930s at Byron Bay on the north coast of New South Wales. Mining of ilmenite began in the mid-1950s near Bunbury in southwest Western Australia. Today, Western Australia is the largest producer. To mine these minerals, sands are dredged through a large suction pipe and the heavy minerals are separated from the lighter sand particles. As the dredge moves slowly forward, the clean sand tailings are pumped back to fill the mined area.
Some higher-grade deposits containing moderately hardened material or layers are mined using equipment such as self-loading scrapers, bucket-wheel excavators, bulldozers and front-end loaders.
Careful environmental rehabilitation of mined areas is carried out progressively as the dredge moves forward. Backfill tailings are shaped to approximate the original landform, then the original topsoil and any overburden is replaced and the area is revegetated, either with local flora or pasture grasses. Environmental monitoring continues as the vegetation matures and the area is eventually rehabilitated, as near as possible, to its previous land use, usually natural bushland or farmland. Public consultation takes place during the approval process prior to consent being given for mine establishment.
Processing
The mined heavy mineral concentrates are sent to 'dry' mills and the individual minerals are separated using their different magnetic and electrical properties at various elevated temperatures. Separation equipment includes high-tension (electrical), high-intensity magnetic and electrostatic plate separators.
Ilmenite is upgraded to synthetic rutile (>90% TiO2) by removing contained iron at plants located at Capel, Geraldton and Muchea, all in Western Australia. The technology used is called the Becher process and was developed by a joint industry and Australian government initiative in the early 1960s. In the Becher process, ilmenite concentrate containing 55%-65% TiO2 (the rest is mainly iron oxide) is fed to a rotary kiln to reduce the iron oxide to metallic iron. Ilmenite grains are converted to porous synthetic rutile grains with metallic iron and other impurity inclusions. The iron is precipitated as hydrated iron oxide from the synthetic rutile grains and a mild acid treatment is used to dissolve the impurities and any residual iron. The grains of synthetic rutile are washed, dried and transported to titanium dioxide pigment manufacturing plants either in Australia or overseas for further processing. An important change to the Becher process at the Geraldton plant has been the development of the Synthetic Rutile Enhancement Process, or SREP, which uses various leaching methods to reduce the level of radioactivity in the synthetic rutile product to internationally acceptable levels.
The Enterprise and the Yarraman mines at North Stradbroke Island in Queensland process their mineral sands ore to produce ilmenite and rutile concentrates. Concentrates are transported by barge to a dry mill plant at Pinkenba near the mouth of the Brisbane River for export through the port of Brisbane.
White titanium dioxide pigment is manufactured at the Kwinana and Kemerton plants in Western Australia. These plants use the chlorination process to produce white pigment by calcining a mixture of synthetic rutile, coke and chlorine to form gaseous titanium tetrachloride (TiCl4). Ilmenite cannot be used as a raw material in the chlorination process because its titanium content is too low. The titanium tetrachloride is condensed to a liquid and most of the impurities separate as solids before it is reheated to a gas and mixed with hot oxygen to form very fine crystalline rutile (raw white pigment). The displaced chlorine gas is recycled to the start of the process. The properties of the raw pigment produced from both pigment processes are enhanced for different uses by coating the crystals with white hydrous oxides of silica, alumina, titania or zirconia (ZrO2).
To obtain pure, white titanium dioxide, the minerals are reacted with chlorine then burned in oxygen. Producing pure titanium metal is only done overseas, where mineral sands are reacted with other chemicals and then heated.