Silver
Last updated:19 April 2024
Introduction
Silver's relative scarcity, attractive appearance and malleability has made it suitable for use in jewellery, ornaments and silverware. It has provided riches for many civilizations, including the ancient Greeks, the Romans, the Tang Dynasty in China, the medieval Saxons and the Spanish and Portuguese in the 16th century.
Today we tend to think of silver only as an ingredient of objects such as cutlery, teapots and jewellery, but it actually has many other uses. In 1813 the first photographic image was made using silver nitrate, and today the main use for silver is in industrial areas - whether in mobile phones or solar panels, new innovations are constantly emerging to take advantage of silver's unique properties. Silver's use has been extensive in coins throughout history, although this application has declined in recent times. In Australia, the 1966 50 cent piece was the last coin in general use to contain silver (80% silver, 20% copper). Although silver is resistant to oxidation it readily forms a surface tarnish of silver sulphide.
Properties
Silver has many special properties that make it a very useful and precious metal. It has an attractive shiny appearance, although it tarnishes easily. The tarnish is silver sulphide and it forms as the silver reacts with sulphur compounds in the atmosphere. Of all the metals, silver is the best conductor of heat and electricity known, in fact it has the highest electrical and thermal conductivity known for any material. It is strong, malleable and ductile and can endure extreme temperature ranges. Silver is also able to reflect light very well.
The Properties of Silver
| Chemical Symbol | Ag (from the Roman word argentum, meaning grey or shining) |
|---|---|
| Ore | Found in native form very rarely as nuggets, but more usually combined with sulfur, arsenic, antimony, or chlorine and in various ores such as argentite, chlorargyrite, and galena (a lead ore often containing significant amounts of silver) |
| Relative density | 10.5 g/cm3 |
| Hardness | 3.25 on Mohs scale |
| Malleability | High |
| Ductility | High |
| Electrical conductivity | Highest known |
| Thermal conductivity | Highest known |
| Melting point | 961°C |
| Boiling point | 2162°C |
Uses
Silver was one of the earliest metals used to make coins. The Romans used silver in this way as early as 269 BC (more than 2000 years ago).
Because silver is such a good conductor of electricity, it is used in many electrical applications, including switches, contacts and fuses. Silver is used in household switch contacts because it does not corrode - corrosion can cause overheating, leading to fire. Although silver tarnishes easily, the tarnish does not prevent the electricity from being conducted. Almost all electrical appliances use silver contacts and switches. Microwaves, dishwashers, TVs, telephones, toys and computers all contain silver. A typical washing machine contains 16 silver contacts.
Silver is an excellent conductor of heat, so one of its uses is in the rear-window defrosters of cars. The tiny silver/ceramic lines conduct heat onto the glass, clearing frost, ice and condensation.
Silver's attractiveness and the ease with which it can be worked make it a popular material for use in jewellery. Sterling silver is an alloy of 92.5% silver and 7.5% copper. Sterling silver is tougher than pure silver, so it can be used to make cutlery, serving trays and other ornaments and decorative tableware. It is also used to make high quality musical instruments such as flutes.
Because silver is so reflective - in fact it is the best reflector known - it is used in mirrors and in coatings for glass or other metals. Metals can be coated with silver by a process called electroplating.
One of the largest uses of silver was in photographic paper and film. Approximately 5000 colour photos can be taken using one ounce of silver. In 1813, Joseph Nicephore Niepce became the first person to create a photographic image using silver nitrate. However, since the inception of digital photography, the use of silver in photography has declined.
The use of silver in medicine is replacing the declining use in photography. Silver has a variety of uses in the health industry. For example, silver sulfadiazine is a very powerful compound used to treat burns and silver is sometimes used in tooth fillings and as a biocide. Bandages with silver ions prevent bacterial growth and speed healing time, making them especially valuable for wound victims.
Silver is used in the manufacture of crystalline solar photovoltaic panels.
Silver is being recycled more and more these days, using scrap photographic materials, electrical equipment, jewellery, chemicals and silverware.
| Use | Description |
|---|---|
| Film | Silver was used heavily in photographic film and paper (mostly as silver bromide), including print, TV, X-ray and video film. |
| Electrical | As silver conducts electricity so well (and it also won't corrode and therefore overheat and cause fires) it is used for switches, contacts and fuses in almost all electrical appliances, from microwaves to dishwashers, TVs to PCs. A typical washing machine contains 16 silver contacts. |
| Soldering | Soldering (often with an alloy of silver, brass and zinc) and welding account for large quantities of silver usage, due to its strength and relatively high melting point. |
| Defrosting | As silver conducts heat so well, it's used in car rear-window de-misting lines, transferring heat onto the window glass to clear it of frost. |
| Jewellery and silverware | Being malleable, ductile and attractive, silver has been used for jewellery and other ornamental pieces since before Roman times. Often it is mixed with copper (7.5%) to make a tougher alloy called 'sterling silver'. |
| Mirrors | Because silver is the most reflective material known, it is used in mirrors. |
| Health | Silver is increasingly being used as a biocide against bacteria in health applications. it is used in some tooth fillings, to make a substance that treats burns, as a wire to keep broken bones still while they heal, in thin plates for bone replacement, for surgical drainage tubes. |
| Money | Silver was one of the earliest metals used to make coins, although today only Mexico has any silver in its coins. In Australia, the last coin to contain silver was the 1966 fifty-cent piece, made from 80% silver and 20% copper. 'Silver' coins are generally now made from nickel and copper. |
| Batteries | Silver batteries are more powerful, smaller and longer-lasting than normal batteries and are therefore especially useful in hearing aids, space satellites and portable TV cameras. |
| Textiles | Silver can be used to prevent bacterial and fungal growth in plastic and textiles. |
History
The use of silver dates from the earliest historic records, it was usually extracted by melting lead ore (galena). The ancient Greeks mined silver at Laurium from the 6th to the 2nd century BC, enabling them to prosper enormously and to build such magnificent structures as the Parthenon and Temple of Poseidon. Silver daggers (alloyed with copper) were found on Crete and date back to the early Minoan period at least 2000 BC. The Romans produced silver from lead-silver mines, smelters and refineries in Britain, Sardinia, Spain and elsewhere and it became the basis of their coinage. The Rio Tinto mine in Spain produced so much silver that the Phoenicians are said to have thrown away their ships' iron anchors and made new ones out of silver, just so they could load more silver on board. In the 16th and 17th centuries, Spain colonised Central and South America, and eventually there was so much silver being mined there that its value went down and gold overtook it as a basis for coinage.
Formation
Many of the metals that are so important to us, such as gold, silver, copper, lead, zinc, are present in the Earth's crust only in very small amounts. Silver is present as only about 5 parts in a million (imagine a million smarties and only 5 of them are silver). Unlike most other base and precious metals, silver is mined and produced mainly as a by-product of the mining of other metals - copper, lead, zinc and, to a lesser extent, gold. There are very few mines around the world in which is silver is the main or only commodity produced, making silver production highly dependent on other commodities.
Apart from gold, all the other metals form compounds with sulphur, called sulphides. Deep in the Earth's crust, where it is very hot, salty water (called brine) circulates and dissolves these metals, collecting them up and concentrating them in the hot brine. The brine can be as hot as 350°C. Sometimes, on the sea floor, this brine comes up through the surface out of holes we call vents. When the hot brine comes into contact with the cold sea water the metal sulphides cannot stay dissolved and precipitate onto the sea floor as various minerals. Copper is precipitated as chalcopyrite (copper sulphide), lead as galena (lead sulphide), and zinc as sphalerite (zinc sulphide). Silver precipitates as a mixture with the other sulphides. The sulphides build up on the seafloor around the vents, like chimneys. Because the water appears black with all the minerals in it, the chimneys are called Black Smokers. Some of these deposits, which are also known as black smokers, are forming today under the ocean off Papua New Guinea, Canada and elsewhere around the world.
Hydrothermal fluids may also be trapped below the surface in cracks where galena containing silver and other minerals may precipitate to make vein deposits. Where limestones occur the fluids may fill cavities to form rich but patchy lead-zinc-silver deposits. For thousands of millions of years, deposits have been forming in these ways and may eventually be exposed at the surface following weathering and erosion. Some are completely eroded away and may be recycled by natural processes into new deposits.
Resources
The main silver minerals are tetrahedrite ((Cu,Fe,Zn,Ag)12Sb4S13), pyragyrite (Ag3SbS3), argentite (Ag2S), proustite (Ag3AsS3) and ceragyrite (AgCl). Australian silver occurs mainly in tetrahedrite, or as coupled (with antimony) substitution in galena (PbS). Silver in gold ores occurs mainly as minor amounts of the natural gold-silver alloy electrum.
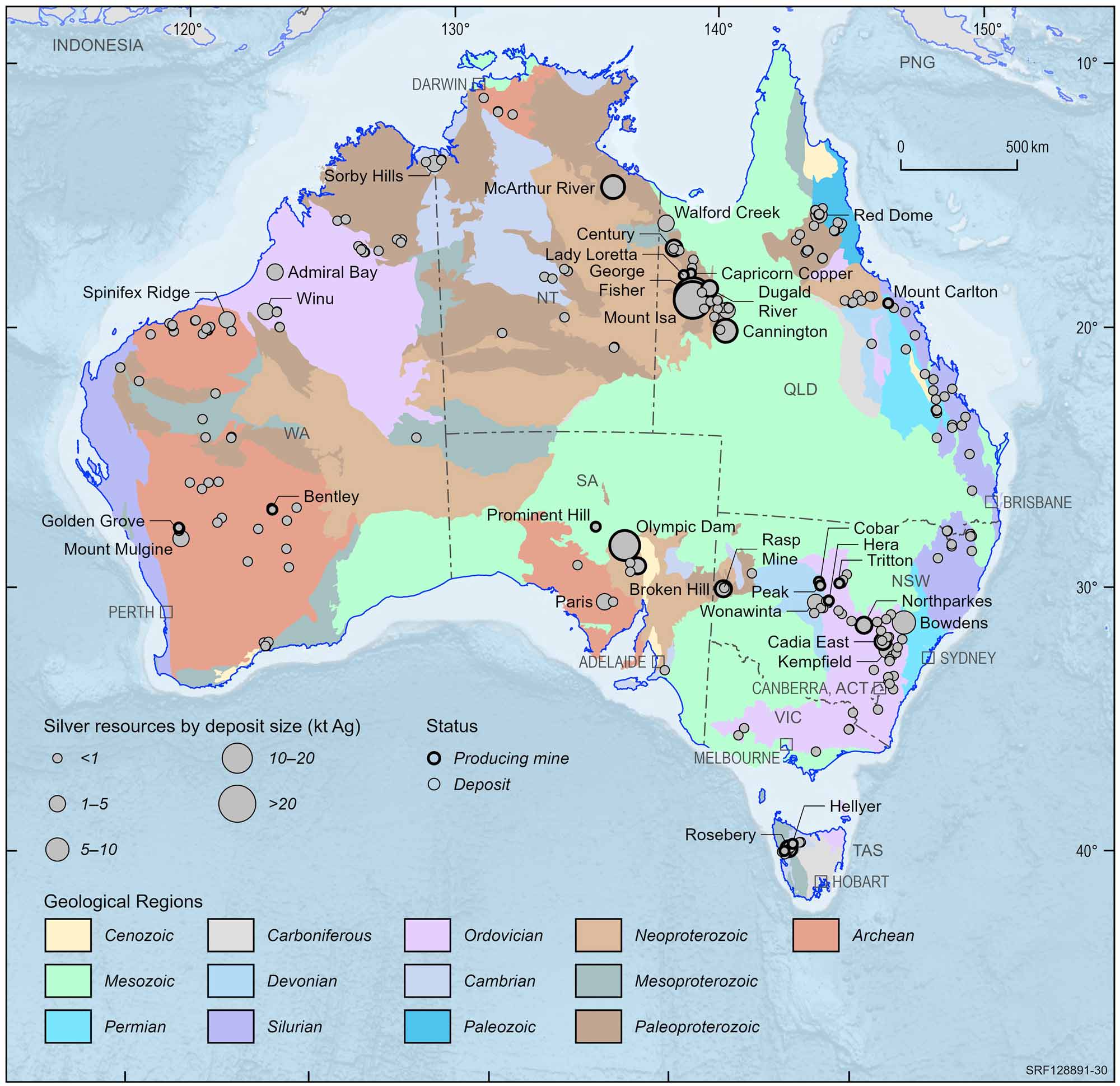
Australian silver deposits and operating mines, 2022.
Deposit size is based on total resources (EDR + Subeconomic Demonstrated Resources + Inferred).
For clarity, only major or significant deposits are labelled.
Silver plays an important part in Australia's history, as the first mine developed in Australia was a silver-lead mine near Adelaide. Partially eroded deposits exposed at the surface were relatively easily discovered. Examples of surface exposure are the deposits at Broken Hill in New South Wales and Mt Isa in Queensland, both of which formed the basis of Australia's silver (lead-zinc) mining industry (see Google Arts and Culture - Broken Hill: one of the world's largest lead-zinc-silver deposits). Exposed deposits are becoming harder to find in Australia and exploration companies are looking beneath the surface for the deposits of the future. This is a more costly and difficult way to find ore bodies although a series of successes have occurred since the late 1970s. Such discoveries include the Scuddles mine (140 metres deep) in Western Australia, the Cannington deposit (10 metres deep) in north Queensland, the Hellyer mine (90 metres deep) in Tasmania and the Wilga deposit in Victoria (50 metres below the surface).
In 1883, Charles Rasp discovered at Broken Hill heavy rocks which he thought may contain tin. Subsequent assays (chemical analysis) of these rocks proved that he had located rich oxidised (weathered) silver and lead minerals. Ore is still being extracted from Broken Hill, which has been the largest producer of lead-zinc-silver in Australia. The wealth generated by mining the Broken Hill ore allowed The Broken Hill Proprietary Company Limited to prosper and, although it no longer has an interest in the deposit, its successor, BHP Billiton has become one of Australia's largest companies.
The upper part of the Mt Isa deposit, which was discovered in 1923 by John Campbell Miles, was also very rich in silver, the prime commodity sought at the time. The rich lead-silver lodes at Mt Isa remain in production after more than 90 years of mining. Discovery of the nearby rich Hilton deposit occurred in the late 1940s but it was not developed until the mid 1980s. In the Mt Isa region, there are large mines at Hilton-George Fisher and Cannington, while the Dugald River and Lady Loretta deposits are yet to be mined. In the Northern Territory, the huge McArthur River lead-zinc-silver deposit is a major producer. The Cannington mine is one of the few mines in which silver is the principal commodity extracted.
Australia has the largest share of the world's economic silver resources, outstripping Mexico, Canada and the USA as a result of the discovery and development of the Mount Isa, Hilton-George Fisher, Cannington and McArthur River lead-zinc-silver deposits. About 25% of Australia's mine output is refined to silver metal and mainly sent to Japan. Most of the remainder is exported in lead bullion to the United Kingdom where it is extracted and refined.
Mining
Almost all of Australia's silver (lead-zinc and/or copper) mines are highly mechanised, underground operations. Ore is drilled and blasted in large volumes and transferred to underground rock crushers by large loaders and trucks. The crushed ore is then hoisted to the surface in skips or driven directly to the surface by truck via a spiral access tunnel (decline). Most of Australia's silver is produced from silver-bearing galena, but some also is produced from copper and gold mining.
Processing
At the surface, the ore is subjected to additional crushing and fine grinding. A flotation process is used to separate the silver-bearing galena and tetrahedrite from other ore minerals and waste rock particles (tailings) to form mineral concentrates. Development of the flotation process occurred in the early days of mining at Broken Hill. Today, more efficient versions of this technology are used world-wide. Many mines around Australia and overseas use the improved Jameson flotation cell, which also was developed in Australia.
In the Jameson process, ground-up ore, water and special chemicals are mixed together and constantly agitated in banks of flotation cells. Air is blown through the mixture in each cell and fine silver-bearing galena particles stick to the bubbles, which rise to form froth on the surface of the cell. The tailings sink to the bottom of the cell and are removed. The froth is skimmed off and the resulting silver-lead sulphide concentrate may assay 800 grams to one kilogram of silver per tonne of concentrate.
The concentrate is sintered (partly melted) to combine the fine particles into lumps and to remove some sulphur as sulphur dioxide. It is then smelted in a blast furnace and drossed (removal of trace copper and impurities) to produce crude lead metal that may contain more than two kilograms of silver per tonne.
Silver is recovered when crude lead is refined to high purity. Crude lead is re-melted and antimony and other impurities removed. The molten lead is then poured into a large upright container called a 'kettle' where it plunges through a molten zinc metal layer floating on top of molten lead. The kettle is increasingly cooled towards its base so that as the incoming molten lead descends, the contained silver and any minor gold or copper combines with the molten zinc dragged down from the zinc layer and forms crystals of zinc-silver-gold-copper alloy. The crystals float to the surface and re-dissolve in the zinc layer. The zinc layer is periodically skimmed off and the silver-gold-copper alloy, called dore, is removed by further smelting then cast into plates for electrolytic removal of copper and separation of high purity silver and gold.
The Port Pirie lead smelter and refinery in South Australia is the most important Australian producer of refined silver and is where lead concentrates from the Broken Hill and other mines are processed. Most of the silver output from Mt Isa and Hilton mines is in lead concentrates, which are smelted to lead bullion at Mt Isa. The bullion is exported to the United Kingdom for refining and silver extraction.
Silver ingots are also produced at the Olympic Dam mine from residues generated during electrolytic copper refining. Silver is extracted and refined by gold refineries in Perth, Kalgoorlie and Melbourne from gold dore or bullion sourced from mainly Australian gold mines.