1.3 What are critical commodities?
This content is being archived
Please be informed that this content is in the process of being archived. Please see our Legacy Publications page for further information.
1.3.1 Definition of critical commodities
The terminology and use of ‘critical’ in the context of raw materials, chemical elements, and minerals is problematic, in part due to the multiple meanings of the word in the English language and also due to the different methodologies used to assess which materials are critical in various studies. The terms ‘critical’ and ‘strategic’ also are used loosely in this context. This report differentiates these terms and focuses only on the critical commodities, although some critical commodities also may be strategic for some countries.
There appears to be a convergence of views, captured in the following quotes from two key studies. The US National Academy of Sciences (2008) report on Minerals, Critical Minerals, and the U.S. Economy proposed that ‘a mineral can be regarded as critical only if it performs an essential function for which few or no satisfactory substitutes exist’, and ‘in addition, a mineral can be regarded as critical only if an assessment also indicates a high probability that its supply may become restricted, leading either to physical unavailability or to significantly higher prices for that mineral in key applications’. In this report the word ‘mineral’ is used in a very broad sense to include individual chemical elements (metals and non-metals) and minerals sensu stricto (see Break-out 1.3.1).
The European Commission (2010) report on Critical raw materials for the EU stated: ‘a raw material is labelled critical when the risks of supply shortage and their impacts on the economy are higher than for most of the other raw materials’.
Break-out 1.3.1. Definitions
Metals are chemical elements that ‘have a characteristic lustre, are good conductors of heat and electricity, and are opaque, fusible, and generally malleable or ductile’ (Neuendorf et al., 2005). Most metals occur in nature as compounds within minerals although some important metals such as gold, copper and platinum also occur naturally in elemental (native) form. Among metals there are several subgroups, including transition metals (such as iron, zinc, copper), noble metals (such as gold, platinum, palladium), alkaline earth metals, etc. Semi-metals have characteristics that are transitional between metals and non-metals, such as semi-conductance of electricity.
A mineral is ‘a naturally occurring inorganic element or compound having a periodically repeating arrangement of atoms, and characteristic chemical composition, resulting in distinctive physical properties’ (Neuendorf et al., 2005).
A mineral deposit is a ‘mass of naturally occurring mineral material, e.g. metal ores or non-metallic minerals, usually of economic value, without regard to mode of origin’ (Neuendorf et al., 2005).
A mineral system is ‘all geological factors that control the generation and preservation of mineral deposits’ (Wyborn et al., 1994).
Both the US and EU studies developed concepts of criticality involving simple 2-dimensional matrices, which express the combination of importance in use and availability or supply risk of the material in question (Figures 1.3.1, 1.3.2). As noted in the introduction, there are many factors contributing to each of these two dimensions—for example, supply risk will be influenced by (1) scarcity of the commodity; (2) geopolitical stability of suppliers; (3) diversity of supply and market scale; (4) method of recovery (e.g., as the main product or as a by-product); and (5) level of concentration of commodity production and processing within particular countries.
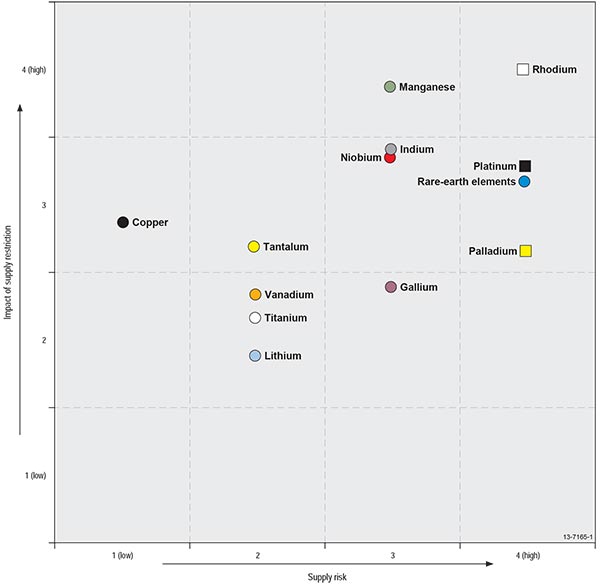
Figure 1.3.1: US National Academy of Sciences (2008) criticality matrix.
This report uses the term ‘commodity’ to cover the wide range of 34 metals, non-metals and minerals studied. These commodities were selected based on an initial review of previous studies of raw materials criticality by different countries. From this list we have assessed the overall criticality, and made judgements about the resource potential for Australia for each commodity (see Section 1.5).
Most of the critical commodities considered in this report are metals and semi-metals; the remainder are non-metallic elements (such as helium, a noble gas), or minerals (such as graphite, a crystalline form of carbon), or rocks (which are composed of aggregates of one or more minerals, such as bauxite).
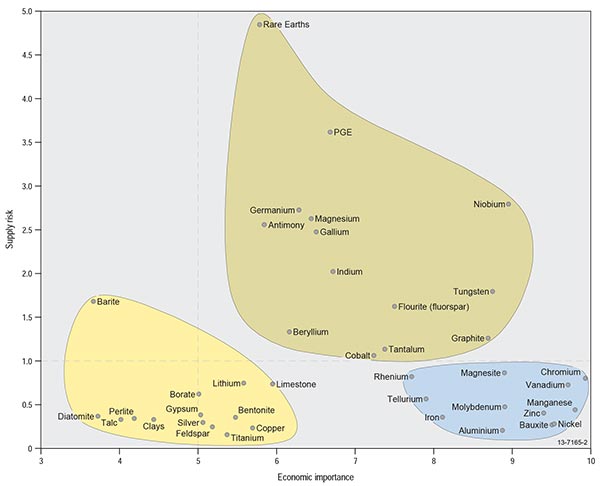
Figure 1.3.2: European Commission (2010) criticality matrix for the European Union.
1.3.2 Uses of critical and other metal and mineral commodities
The periodic table of the elements (Figure 1.3.3) illustrates the groupings of elements with certain shared physical and chemical properties. For example, all metals are good conductors of electricity and are generally malleable and ductile, whereas semi-metals are semi-conductors of electricity, a highly valuable property in electronics and solar energy panels. Some sub-groups have particular shared properties, for example platinum-group elements (including platinum and palladium) and other noble metals such as gold are highly resistant to chemical corrosion.
Other metals are valued for their extremely high melting temperatures and hardness, such as tungsten and rhenium, so that alloys of these metals tend to have greater tensile strength at high temperatures. This property enables rhenium-bearing super-alloys in jet engine turbine blades to operate at higher temperatures than non-rhenium turbines (break-out 1.3.2), reducing aeroplane emissions and fuel costs.
The rare-earth elements, which include the lanthanide series metals as well as scandium and yttrium, have diverse and very useful properties. For example, small percentages of neodymium and dysprosium in some alloys increase permanent magnet strength by orders of magnitude, enabling step changes in miniaturising of telecommunications and other electronic devices, and much more efficient generation of electricity in commercial wind turbines.
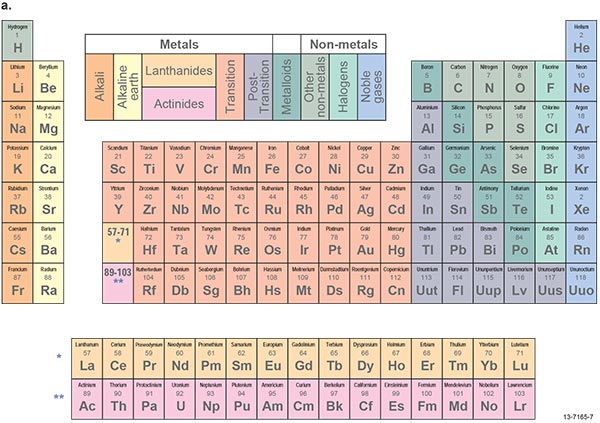
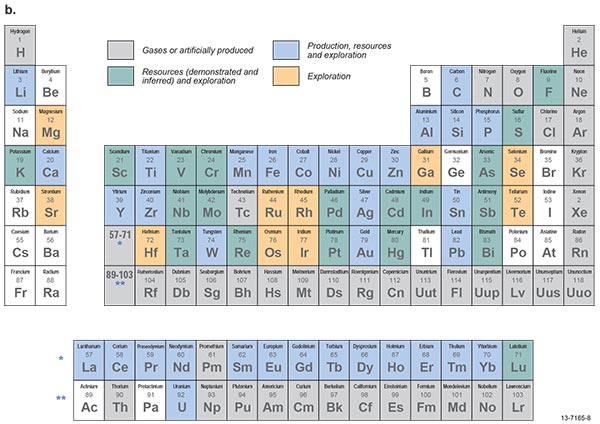
Figure 1.3.3: Periodic tables of the elements showing (a) metals, semi-metals (metalloids) and non-metals with sub-groups, and (b) the status of production, development and exploration in Australia.

Image: Tony Hisgett,
Wikimedia Commons.
| Operating Property - testing this table | Non-Rhenium Turbine | Rhenium Turbine |
|---|---|---|
| *Source Rio Tinto December 2008 Review. 1 The values X and Y refer to nominal power and thrust and efficiency for non-rhenium turbines. The formulae for rhenium turbines indicate improvements in these parameters. |
||
| Operating temperature | ˜2000–2200° F | ˜3000° F |
| Power and thrust | X1 | Approximately 2X |
| Fuel Efficiency | Y1 | Y + (40–60%) |
| Emissions* | Carbon dioxide | 64% reduction |
| Nitrogen oxide | 88% reduction | |
| Sulphur dioxide | 99.9% reduction | |
| Particulates | Eliminated | |
| Approximate Rhenium contained per turbine | 0 kg | ˜25 kg (3% alloy) |
Table 1.3.1 lists some of the key drivers of the technologies in which major as well as minor commodities are used, many of which are considered critical (see Sections 1.4, 1.5). The commodities assessed in this report are shown in bold. Break-out 1.3.3 illustrates the use of mineral commodities in the construction of a typical motor car. Other drivers are the production and use of low-emissions energy, which is likely to see extensive growth in the short, medium and long terms, and the communications and entertainment technology industries.
| Driver of metal/material usage | Technology/product | Commodities used; bold indicates critical commodities in this study |
|---|---|---|
| Industrial production efficiency and infrastructure development | Steel | Fe, Cr, V, Mo, Ni, Co, Mn |
| Catalysts | PGE (Pt, Pd) | |
| Ceramics | Li, Ce | |
| Paint | Ti, Cr | |
| Moulds | Zr | |
| Flame retardant | Sb | |
| Cryogenics | He | |
| Low-emissions energy production | Wind turbines—permanent magnets | REE (Nd, Dy, Sm, Pr) |
| Photo-voltaics (PV) | In, Sb, Ga, Te, Ag, Cu, Se | |
| Nuclear reactors | U, Th, Zr | |
| Low-emissions energy usage | Electric cars—batteries | REE (La, Ce, Nd, Pr), Li, Ni, Co, Mn, graphite |
| Electric cars—magnets | REE (Nd, Dy, Sm, Pr) | |
| Electric cars—fuel cells | PGE, Sc | |
| Cars—light metals | Al, Mg, Ti | |
| Cars—catalytic converters | PGE | |
| Communications and entertainment technologies | Wires | Cu |
| Micro-capacitors—mobile phones etc | Ta, Nb, Sb | |
| Flat screens—phosphors | In, Y | |
| Fibre optics and infra-red | Ge | |
| Semiconductors | Ga | |
| Defence / security | Nuclear/radiation detectors | He |
| Armour and weapons | Be, W, Cr, V | |
| Aerospace—superalloys | Re, Nb, Ni, Mo | |
| Transport—fuel efficiency & performance | Light alloys Superalloys (high-temperature performance e.g. in jet engine turbines) High speed trains—magnets | Al, Mg, Ti, Sc, Th Re, Nb, Ni, Mo Co, Sm |
| Water & food security | Water desalination | PGE, Cr, Ti |
| Agricultural production—fertiliser | Phosphate rock; potash, Mg |

Images:
(a) http://flickr.com/people/robadob,
Wikimedia Commons,
(b) Larry D. Moore,
Wikimedia Commons,
(c) Petter73, Wikimedia Commons.
| Element/material | Mass (kg) | Property (use) |
|---|---|---|
| Iron and steel | 963 | High strength, durability (frame, engine) |
| Aluminium | 109 | Light weight (frame, engine) |
| Carbon | 23 | Bond strengthener (tyres and other rubber parts) |
| Copper | 19 | Electrical conductivity (wiring) |
| Silicon | 19 | Bonding properties (windows) |
| Lead | 11 | Conductor (storage batteries) |
| Zinc | 10 | Galvaniser, strengthener (galvanised metal and alloy parts) |
| Manganese | 8 | Hardness as metal alloy parts |
| Chromium | 7 | Corrosion resistance and hardness as metal alloy |
| Nickel | 4 | Strength at elevated temperature and corrosion resistance as metal alloy |
| Magnesium | 2 | Light weight alloying element with aluminium |
| Sulfur | 0.9 | Strengthens rubber tyres |
| Molybdenum | 0.45 | Strength and toughness as metal alloy |
| Vanadium | <0.45 | Strength and toughness as metal alloy |
| Platinum | 1.5–3.0 grams | Catalytic properties (catalytic converters) |
| Note: In addition to the elements listed above, the average car also contains trace amounts of antimony, barium, cadmium, cobalt, fluorspar, gallium, gold, graphite, halite, limestone, mica, niobium, palladium, phosphorus, potash, strontium, tin, titanium and tungsten. | ||




